Physics for Information Science: Unit I: Electrical Properties of Materials
Particle in a Three Dimensional Potential Box
Electrical Properties of Materials
The solution of one-dimensional potential box can be extended for a three dimensional potential box. In a three dimensional potential box, the particle (electron) can move in any direction in space.
PARTICLE IN A THREE
DIMENSIONAL POTENTIAL BOX
The
solution of one-dimensional potential box can be extended for a three
dimensional potential box. In a three dimensional potential box, the particle
(electron) can move in any direction in space. Therefore instead of one quantum
number 'n', we have to use three quantum number nx, ny
and nz corresponding the three co-ordinate axis (ie) x, y and z
respectively.
Particle in a three dimensional potential box
Let
us consider a particle enclosed in a 3-dimensional potential box of length a, b
and c along x, y and z axis respectively as shown in fig 1.7.
Since
the particle inside the rectangular box has elastic collisions with the walls,
the potential energy of the electron inside the box is constant and can be
taken as zero for simplicity.
We
can say that outside the box and on the wall of the box, the potential energy
is ∞.
The
boundary conditions are
To
find the wavefunction of the particle within the boundary conditions (1). Let
us consider the 3-dimensional schrodinger time independent wave equation,
i.e.,
............(3)
Since
V=0 [For a free particle], we can write eqn (3) as
............(4)
Equation
(4) is a partial differential equation, in which Ψ is a function of
three
variables, x, y and z.
We
can solve this using method of separation of variables.
The
solution for eqn (4) can be written as
Ψ
(x, y, z) = X (x) Y (y) Z (z)
Which
means Ψ is a function of x, y and z and
is equal to product of 3 functions i.e., X, Y and Z.
Where
X is a function of x only
Y
is a function of y only
and
Z is a function of z only
We
can write the solution for equation (4) as
Ψ = XYZ ….(5)
Differentiating
eqn (5), Partially with respect to 'x', twice, we get
............(6)
Similarly
differentiating eqn (5) partially with respect to 'y', twice, we get
............(7)
Similarly
differentiating eqn (5) partially with respect to 'z', twice, we get
............(8)
Substituting
equations (5), (6), (7) and (8) in eqn (4) we get
Dividing
by XYZ on both sides we get
............(9)
(or)............(10)
where............(11)
In
equation (10), L.H.S. is independent of each other and is equal to a constant
in R.H.S. we can equate each term of L.H.S. to each constant in R.H.S.
We
can write
or)............(12)
(or)............(13)
(or)............(14)
Equations
(12), (13) and (14) represents the differential equations in
x,
y and z co-ordinates. The solution for equation (12) can be written as
X (x) = Ax sinkx
x + Bx cos kx x ....(15)
where
A and B, are arbitrary constants, which can be found by applying boundary
conditions.
i.e.,
(i) When x=0; X=0
Equation
(15) becomes
0=0+Bx
Bx=0
.....(16)
(ii) When x = a; X=0
Equation
(15) becomes
0
= Ax sinkx a
Here
Ax ≠ 0
[Because,
if Ax = 0, then X (x) becomes zero, which implies that the particle.
is not there, and is meaningless]
sin
kx a = 0
We
know sin nx π = 0
Comparing
the above two equations we can write kx a = nx π
(or)............(17)
Substituting
equations (16) & (17) in eqn (15) we get
............(18)
Equation
(18) represents the un-normalized wave function
Normalization
Eqn (18) can be normalized by integrating it within the limits i.e., boundary conditions 0 to a,
(or)............(19)
Substituting
eqn (19) in (18) we get
............(20)
Similarly
by solving equation (13) and equation (14) with the boundary conditions 0 to b
and 0 to c respectively, we can write
............(21)
............(22)
Eigen functions
The
complete wave function, for equation (4) can be written as
Ψ
(x, y, z) = X (x) Y (y) Z (z)
Substituting
equations (20), (21) and (22) in the above equation, we get
............(23)
Equation
(23) represents the eigen function for an electron in a rectangular box.
Eigen values
From
equation (11) we can write
............(24)
From equation (17) we can write
similary we can write
Substituting
these values in eqn (24), we get
(or)............(25)
Equation (25) represents the energy eigen values of an electron in a rectangular box.
Cubical box
For
a cubical box, a = b = c,
We
can write equation (25) as
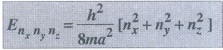 ............(26)
............(26)
The
corresponding normalized wave function of an electron in a cubical box can be
obtained from equation (23), as

From
equations ……(26) and ….(27) we can note that, several combinations of the three
quantum numbers (nx, ny, and nz) leads to
different energy eigen values and eigen functions.
Example
If
a state has quantum numbers nx = 1; ny = 1; nz
=2
Then,
nx2+ny2+nz2 =
6
Similarly
for nx = 1; ny = 2; nz = 1 combination and nx
= 2; ny = 1; nz = 1 combination we have nx2+ny2+nz2
= 6
 ............(28)
............(28)
The
corresponding wave functions can be written as
 ............(29)
............(29)
Physics for Information Science: Unit I: Electrical Properties of Materials : Tag: : Electrical Properties of Materials - Particle in a Three Dimensional Potential Box
Related Topics
Related Subjects
Physics for Information Science
PH3256 2nd Semester CSE Dept | 2021 Regulation | 2nd Semester CSE Dept 2021 Regulation
